- Have Any Question?
- (+880) 017238012739
- enviroco@gmail.com
Reverse Osmosis
Reverse Osmosis (abbreviation: RO), also called hyperfiltration, is the process in which the passage of solvent particles from the more concentrated solution to the less one is forced by applying a pressure greater than the osmotic pressure to the more concentrated solution.
In practice, a membrane is used to perform Reverse Osmosis. This membrane keeps the solute on one side and prevents it from moving, allowing pure solvent to come from the other. As much mechanical work is required to cancel the effect of osmotic pressure as is necessary, this phenomenon is not spontaneous.
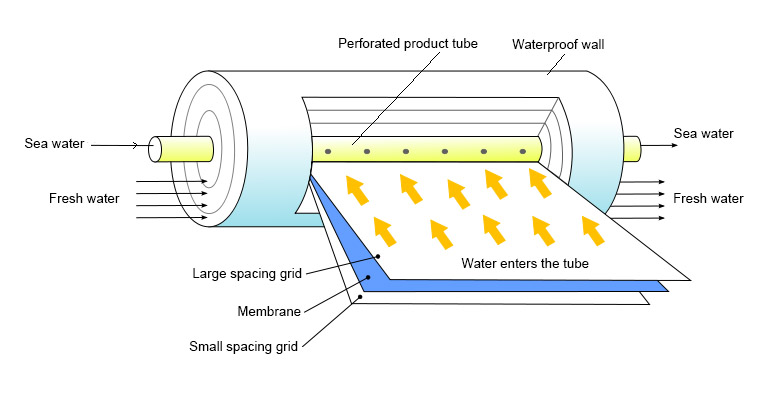
This method is the best way to filter water because it doesn’t just have a physical barrier that is determined by the size of the pore. Instead, it uses the different chemical bonds that different species have with the membrane to let molecules like short-chain alcohols, which are chemically similar to water, through. Plant-wise, the process utilizes the guideline of tangential filtration, as well as other membrane separation techniques like microfiltration, ultrafiltration and nanofiltration.
Desalination and the removal of trace amounts of phosphates, calcium, heavy metals, phytochemicals, radioactive materials, and nearly all polluting molecules are two of the water treatment processes for what the reverse osmosis is used.
Lately, “zero liquid discharge" plants are built in which the Reverse Osmosis section elevate the concentration of chemical species in the wastewater to valors near or over their solubility (over-soaked arrangements).
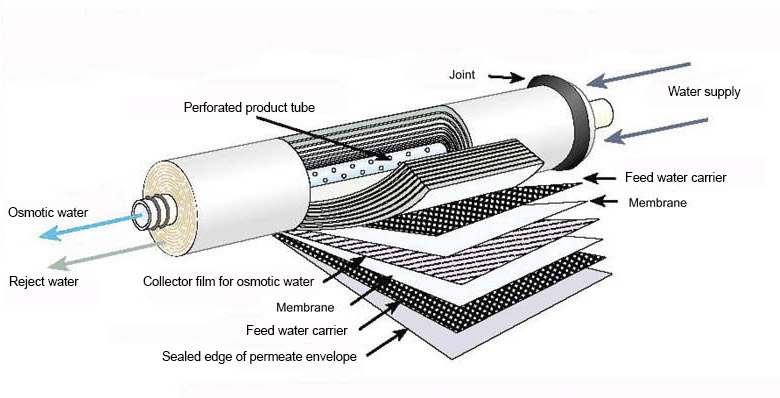
The Reverse Osmosis process makes use of thin film composite membranes (TFC or TFM). These semipermeable membranes are primarily designed for use in systems for water purification or desalination, but are also used in chemical industries like batteries and power devices.
A TFC material is, in essence, a molecular sieve made of a film of two or more layered materials.
Polyamide, which is chosen for its water permeability and relative impermeability to various dissolved impurities, such as salt ions and other small molecules that cannot be filtered out, is typically used to make membranes for RO. The dialysis membrane is another example of a semipermeable membrane.
Complete the following form and tell us about your project. We will contact you as soon as possible
